Sophism-free Atomic Model
Looking at the currently accepted atomic model I was surprised to learn that an important element is completely ignored - electron is in constant movement thus as a charged particle is must produce some magnetic field. Let’s take a look how it will affect our understanding of the atom.
As you know since high school, there is an interaction between two charged particles if they move towards or away from each other generate two magnetic fields, if the particles are of same charge, charges repulse them but magnetic fields attract, inversely if charges are opposite they attract but induced magnetic fields repel. This principle becomes very handy explaining why negatively charged electrons and positively charged nuclei do not collapse.
Imaging, an isolated hydrogen nucleus and an electron separated by some distance, they are mutually attracted thus the approach begins. Their relative velocity increases so does repelling them magnetic fields. Those fields prevent them from collision; instead the electron is trapped by the nucleus and starts orbiting it.
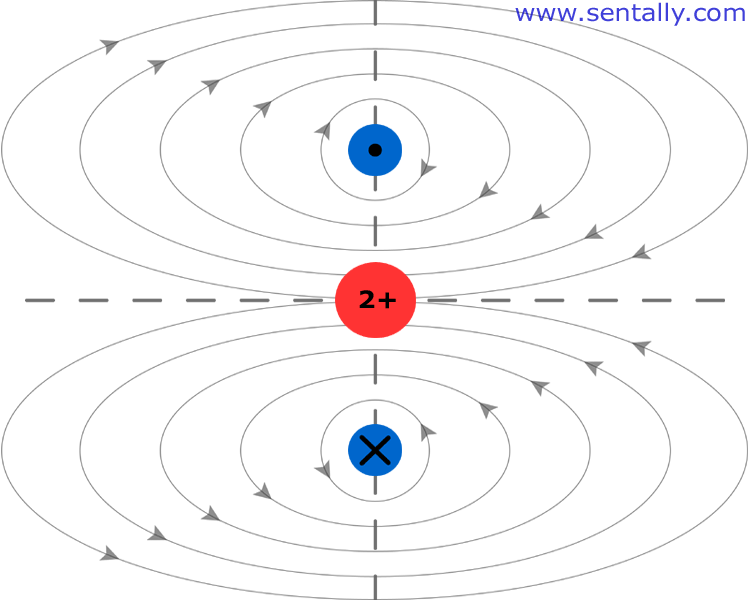
What happens to the electron then? Depicted below helium atom has a nucleus containing two positively charged protons and two negatively charged electron rotating around it. Conventionally, we marked one electron with a dot it goes out of the page and second electron marked with x goes into the page, magnetic fields generated by the electrons represented by lines. Combined magnetic fields create magnetic dipole of the atom, in plane English – every atom is a magnet with north and south poles, so far it was consistent with quantum electromechanical model.
Contrary to quantum physics postulates we can determine a position of electron at least relatively to the other. In the helium atom electrons tend to be at the opposite sides because of the negative charges they both have.
Also electrons rotate on the same orbit and keep same direction. It is still possible to rotate in opposite direction, but the orbits must be different and curbed, in this case the atom will lose its magnetic dipole. If we can detect an atom without magnetic dipole, it will be it.
Interesting things happen when an atom has other then two electrons. For example, hydrogen atom always forms some chemical compound to reach equilibrium, because with one electron atom is energetically unstable or “exited” - the term borrowed from quantum physics. The animated picture shows a carbon atom with six electrons placed on two parallel orbits, for better visibility I used two semitransparent planes. Several forces acting in that system:
- Magnetic field created by rotating electrons makes those electrons to rotate in same direction and with nucleons’ electric dipoles responsible for keeping nucleus from disintegrating - it is what scientists call "strong force" and still struggling to explain it
- Repelling force between electrons (due to negative charges) holds them as far as possible. On the picture electrons are evenly distributed on two parallel orbits
- Centrifugal force acting on electrons holds them away from the nucleus and tends to approach and merge the two orbits
- Attraction between electrons and protons keeps electrons orbiting without escaping
- All electrons have equal angular velocity, regardless of the orbit radii
- Impact of the gravitational force is negligible
I intentionally chose a carbon atom it is easy to show the six electron distribution. But what happens if we have more of them? Additional electrons are placed on existing orbits, orbits respected radii are increased and more orbits formed if needed.
It is considered that the strongest chemical bond formed by covalent electrons, looks like it is formed by magnetic dipoles, electrons readjust their orbits afterwards.
Redefining the nature of light
According to the described model, each absorption or release of energy, each collision on atomic or subatomic level will cause sudden change in the magnetic field; witch will send out an electromagnetic wave (light, heat, X-ray etc.). And here the “heresy” begins, photon is emitted not only when the atom looses its energy but when it absorbs energy as well.